
#Cobalt electron configuration spy free#
Melting and boiling point: Cobalt has a high melting and boiling point because of free electrons and strong metallic bonds.Conductivity: Cobalt has high conductivity due to the large number of valence electrons in the outer shell.The significance of cobalt’s electron configuration in physical properties includes: What are the implications of cobalt’s electron configuration on its chemical properties? Since cobalt exhibits various oxidation states, knowing electron configuration also helps to understand their properties in detail.The element’s positions in the periodic table, where they belong in s,p,d,or f blocks can be predicted.Physical properties of cobalt can be determined from the electron configuration like boiling point,melting point,density etc.The valence electron in the atom gives us the details of the chemical behavior of the cobalt element.Cobalt can control the charge flow in the lithium battery because of its properties helpful for working of the battery.The scientist can easily find out the number and details of how electrons are arranged around a nucleus.Knowing the electron configuration of cobalt is significant because it accounts for the chemical and physical properties of cobalt atoms. What is the importance of knowing the electron configuration of cobalt? The electron configuration of Co 2+ is 1s 2 2s 2 2p 6 3s 2 3p 7 3d 6 as it loses two electrons from the 4s subshell. What is the electron configuration of cobalt ion? Thus,the electron configuration of Cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. Now, electrons get filled in each subshell as one electron fills first then only the second electron gets paired.Two electrons get filled in 1s subshell and then 2s having higher energy than 1s orbital.The Hund’s rule states that each subshell in an orbital is occupied by a single electron before any subshell is occupied by two electrons and the single electron occupied in an orbital has the same spin.

The electron configuration can be filled in the orbitals using Hund’s rule.

Thus, the electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7.
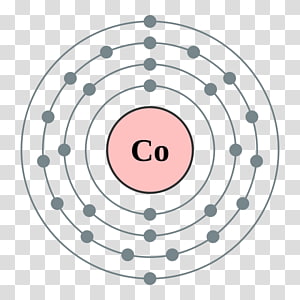
Cobalt atom requires three more valence electrons in its d-orbital to attain noble gas configuration. The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. What is the electron configuration of cobalt? What are the implications of cobalt’s electron configuration on its physical properties?.What are the implications of cobalt’s electron configuration on its chemical properties?.What is the importance of knowing the electron configuration of cobalt?.What is the electron configuration of cobalt ion?.How can we find the electron configuration of cobalt?.What is the condensed electron configuration of cobalt?.The electron Configuration Diagram of cobalt.What is the complete electron configuration of cobalt?.What is the electron configuration of cobalt?.


 0 kommentar(er)
0 kommentar(er)
